
Fifty shades of “nanos”
Since the first definition of “nanoparticle” made its appearance in the scientific and regulatory landscapes, a myriad of new terms related to “nano” have popped up. Everything nowadays could be “nano”. Here we want to focus on a few concepts that, for different reasons, are relevant today, without aspiring to provide a thorough compendium of the subject but to offer you some (nano)food for thought.
WHAT A NANOMATERIAL IS
It has already been a while since the European Commission (EC) recognised an important lack in definitions, pointing out “the absence of generally accepted definitions for the term nanomaterials1” (NMs). It was 2008. From that moment, a process started to get a common, harmonised and cross-cutting definition and the European Parliament asked the Commission to establish a “comprehensive science-based definition of nanomaterials in Community legislation as part of nano-specific amendments to relevant horizontal and sectoral legislation”. The result was the Recommendation 2011/696/EU2 on the definition of “nanomaterials” for regulatory purposes. It was intended to be used by the Member States, European Union agencies and companies, also by the Commission in EU legislation and implementation instruments and ensuring a consistent regulatory approach (COM(2012) 572 final)3. From that first effort, a lot has been written around the term “nanomaterials”, either by recognised institutions or other uncertified sources, without achieving a unanimous consensus on a definition that meets the requirements demanded by the regulatory context. It should be noted that nanomaterials are covered by the “substance” definition of the REACH Regulation (Art. 3)4, the overarching legislation is applying to chemical substances.
Ten years after that historical recommendation, the European Commission has recently called for a “targeted stakeholder consultation relating to the review of the EU recommendation on the definition of the term “nanomaterial”5, which is one of the actions announced in the 2020 Commission Chemicals Strategy for Sustainability released under the Green Deal.
Who are then the targeted stakeholders?
In the EU official text of the survey, we read: “economic operators implementing all relevant EU sectoral regulation and their federations, Member States competent authorities and other regulatory stakeholders, research organisations supporting implementation, academia and NGOs.”
Are you a targeted stakeholder? Would you like to give your contribution to the European harmonisation process?
You still have time! Participate until 30th June 2021 using the link below, or you will have to wait another five years for this opportunity, as the Commission has committed itself to review the definition again by December 2026. The review is an iterative process in which practical experience, scientific and technological findings will play an important role in the further development of methods and standards.
WHAT ABOUT NANOMATERIALS IN PRODUCTS?
In our daily life, we accidentally come in contact very often with nanomaterials as they are used as a key component of several commercial products.
Are also those products nanomaterials?
Based on the EC Recommendation’s scope, we should name nanomaterials those objects meeting the Recommendation requirements, when they are substances or mixtures, but implicitly not when they are final products. This means that if a nanomaterial is used as an ingredient amongst other components, the entire product will not become a nanomaterial rather a “Nano-enabled product” (NEP). This concept is becoming more and more relevant because of the potential implications for environmental and human health due to the potential release in the environment of engineered NMs from the final products. What about the regulatory aspects so far? Under REACH, three terms are used officially: substances, mixtures and articles. Nano-enabled products therefore may be covered by the Art. 3 REACH4 definition of an article, “an object which during production is given a specific shape, surface or design which determines its function to a greater degree than does its chemical composition”.
Since different engineered NMs have different physicochemical and toxicological properties, further efforts are required to help the classification and prioritisation of NEPs for environmental risk profiling.

ONE, NO ONE AND ONE HUNDRED THOUSAND
It is important to point out that each nanomaterial can also exist in multiple forms due to the various ways in which particles can be manipulated. For example, different synthesis processes can generate materials with the same chemical composition, but different shape or deliberate changes can be made to the surface of the same kind of particles. How to refer then to each of these variations? Based on the 2011 EC Recommendation, Annex VI of the REACH Regulation and dedicated ECHA guidance documents define “nanoforms” or a set of similar nanoforms as “forms of a natural or manufactured substance containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50 % or more of the particles in the number size distribution, one or more external dimensions is in size range 1-100 nm (including also by derogation fullerenes, graphene flakes and single-wall carbon nanotubes with one or more external dimensions below 1 nm) differing by size distribution, shape and other morphological characterisation, surface treatment and functionalisation and specific surface area of the particles6.
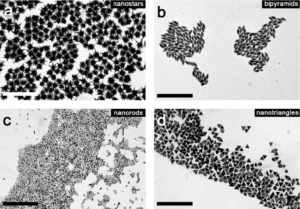
The concepts and terms used in this context follow the concepts and terms used in the European Commission’s recommendation on the definition of nanomaterial. It is important for stakeholders involved in the manufacturing or import of nanoform(s) of a substance to be constantly updated on the evolution of the concept and on the requirements to comply with their obligations under the REACH Regulation. To help duty holders under REACH, several nano-specific appendices to ECHA guidance documents have been released by ECHA as a complement of the existing guidance documents, i.a. the guidance on registration7 or several appendices to the R-series guidance on chemical safety assessment, as well as most recently a practical guide on how to prepare registration dossiers covering nanoforms.
Those of you interested in learning more about the subject can visit the ECHA website in the section dedicated to nanoforms https://echa.europa.eu/it/-/updated-guidance-for-registering-substances-in-nanoform.

Here is the link to participate in the “Targeted stakeholder consultation relating to the Review of the EU recommendation on the definition of the term “nanomaterial”:
EUSurvey – Survey (europa.eu).
Written by: Elisa Moschini, Luxembourg Institute of Science and Technology (LIST), Luxembourg.
REFERENCES
1First Regulatory Review on Nanomaterials SEC(2008) 2036.
2Recommendation 2011/696/EU on the definition of nanomaterial.
3Second Regulatory Review on Nanomaterials COM(2012) 572 final.
4Art. 3 (2) Definitions and general provisions. Regulation (EC) No. 1907/2006 (“REACH”).
2European Commission (May 2021). “Targeted stakeholder consultation relating to the Review of the EU recommendation on the definition of the term “nanomaterial”.
6 Annex VI to Regulation (EC) No. 1907/2006 (“REACH”); Appendix for nanoforms applicable to the Guidance on Registration (03/12/2019) and guidance on substance identification (03/12/2019).
7ECHA practical guide (April 2021) How to prepare registration dossiers covering nanoforms; ECHA guidance on information requirements and chemical safety assessment (Nanomaterial appendix to R.6, R.7, R8, R10, R14).

